The secret life of teeth: Evo-devo models of tooth development

A simple, straightforward developmental rule — the “patterning cascade” — is powerful enough to explain the massive variability in molar crown configuration over the past 15 million years of ape and human evolution. Photo courtesy Pixabay.com
Across the world of mammals, teeth come in all sorts of shapes and sizes. Their particular size and shape are the process of millions of years of evolutionary fine-tuning to produce teeth that can effectively break down the foods in an animal’s diet. As a result, mammals that are closely related and have a similar menu tend to have teeth that look fairly similar. New Arizona State University research suggests, however, that these similarities may only be “skin deep.”
The teeth at the back of our mouths — the molars — have a series of bumps, ridges and grooves across the chewing surface. This complex dental landscape is the product of the spatial arrangement of cusps, which are conical surface projections that crush food before swallowing. How many cusps there are, how they are positioned and what size and shape they take together determine a molar's overall form or configuration.
Over the course of hominin (modern humans and their fossil ancestors) evolution, molars have changed markedly in their configuration, with some groups developing larger cusps and others evolving molars with a battery of smaller extra cusps.
Charting these changes has yielded powerful insights into our understanding of modern human population history. It has even allowed us to identify new fossil hominin species, sometimes from just fragmentary tooth remains, and to reconstruct which species is more closely related to whom. Exactly how some populations of modern humans, and some fossil hominin species, evolved complex molars with many cusps of varying sizes, while others evolved more simplified molar configurations, however, is unknown.
In a study published this week in Science Advances, an international team of researchers led by ASU’s Institute of Human Origins and School of Human Evolution and Social Change found that a simple, straightforward developmental rule — the “patterning cascade” — is powerful enough to explain the massive variability in molar crown configuration over the past 15 million years of ape and human evolution.
“Instead of invoking large, complicated scenarios to explain the major shifts in molar evolution during the course of hominin origins, we found that simple adjustments and alterations to this one developmental rule can account for most of those changes,” said Alejandra Ortiz, a postdoctoral researcher with the Institute of Human Origins (IHO) and lead author of the study.
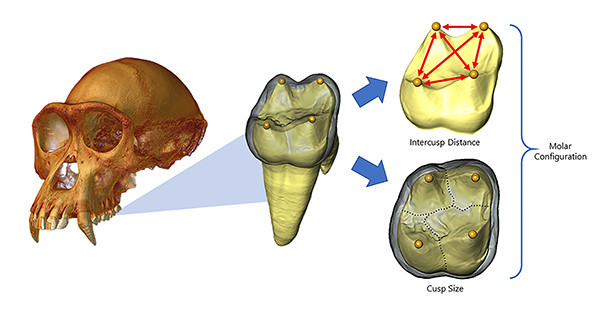
CT-rendered chimpanzee cranium (left) with enlarged image of a virtually extracted molar (middle). The outer layer, called enamel, is rendered transparent revealing the 3-D landscape of a molar’s underlying dentine core. The location of embryonic signaling cells that will determine future cusp position is indicated by yellow spheres (middle). The distribution of these signaling centers across the dentine landscape is measured as a series of intercusp distances (red arrows in right, top), which determines the number of cusps that will ultimately develop across a molar crown, as well as the amount of terrain mapped out by each cusp (dashed lines in right, bottom). Image credit: Alejandra Ortiz and Gary Schwartz
In the past decade, researchers’ understanding of molar cusp development has increased a hundredfold. They now know that the formation of these cusps is governed by a molecular process that starts at an early embryonic stage. Based on experimental work on mice, the patterning cascade model predicts that molar configuration is primarily determined by the spatial and temporal distribution of a set of signaling cells.
Clumps of signaling cells (and their resultant cusps) that develop earlier strongly influence the expression of cusps that develop later. This cascading effect can result in either favoring an increase in the size and number of additional cusps or constraining their development to produce smaller, fewer cusps. Whether this sort of simple developmental ratchet phenomenon could explain the vast array of molar configurations present across ape and human ancestry was unknown.
Using state-of-the-art microcomputed tomography and digital imaging technology applied to hundreds of fossil and recent molars, Ortiz and her colleagues created virtual maps of the dental landscape of developing teeth to chart the precise location of embryonic signaling cells from which molar cusps develop. To the research team’s great surprise, the predictions of the model held up, not just for modern humans, but for over 17 ape and hominin species spread out across millions of years of higher primate evolution and diversification.
“Not only does the model work for explaining differences in basic molar design, but it is also powerful enough to accurately predict the range of variants in size, shape and additional cusp presence, from the most subtle to the most extreme, for most apes, fossil hominins and modern humans,” Ortiz said.
These results fit with a growing body of work within evolutionary developmental biology that says very simple, straightforward developmental rules are responsible for the generation of the myriad complexity of dental features found within mammalian teeth.
“The most exciting result was how well our results fit with an emerging view that evolution of complex anatomy proceeds by small, subtle tweaks to the underlying developmental toolkit rather than by major leaps,” said Gary Schwartz, a study coauthor, paleoanthropologist with IHO and associate professor with the School of Human Evolution and Social Change.
This new study is in line with the view that simple, subtle alterations in the ways genes code for complex features can result in the vast array of different dental configurations that we see across hominins and our ape cousins. It is part of a shift in our understanding of how natural selection can readily and rapidly generate novel anatomy suited to a particular function.
“That all of this precise, detailed information is contained deep within teeth,” continued Schwartz, “even teeth from our long-extinct fossil relatives, is simply remarkable.”
“Our research, demonstrating that a single developmental rule can explain the countless variation we observe across mammals, also means we must be careful about inferring relationships of extinct species based on shared form,” said Shara Bailey, a coauthor and paleoanthropologist at New York University. “It is becoming clearer that similarities in tooth form may not necessarily indicate recent shared ancestry,” added Bailey, who, in 2002, was the first doctoral graduate to be affiliated with IHO.
More Science and technology

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity…

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State…

