Switched-on bacteria: new RNA regulatory system found in Salmonella
Disease-causing bacteria possess elaborate defensive arsenals used to withstand the body’s best efforts to annihilate them. Salmonella, causative agent of food-borne illness, is one such pathogen, displaying remarkable abilities to keenly sense and respond to environmental cues.
In a new study, Yixin Shi, Guang Zhao and Wei Kong, researchers at Arizona State University’s Biodesign Institute and School of Life Sciences, have found a new RNA regulatory factor in Salmonella (and perhaps other pathogenic bacteria) used to subtly calibrate levels of manganese ions.
This RNA-mediated control – known as a riboswitch – senses and responds to manganese, allowing more of the ions into the bacterial cell when concentrations are low, and shutting off the manganese faucet when ion concentrations become too high.
Manganese plays a critical role in Salmonella’s ability to infect cells and survive efforts by the host defense system to eradicate it. Future therapies targeting and disabling the manganese riboswitch in Salmonella (or similar riboswitches in other invasive pathogens) could provide clinicians with a silver bullet – one lethal to pathogenic invaders, yet harmless to the host.
Techniques drawing on riboswitch research may prove especially useful for reversing antibacterial drug resistance in a range of infectious microbes.
The group’s research findings recently appeared in the Journal of Biological Chemistry.
RNA today
Ribonucleic acid, or RNA, was once thought to play a purely intermediary role in the genetic regime, dutifully copying the commands laid down in the DNA sequence, which is first transcribed into RNA, then translated (with the help of ribosomes) into a functional protein.
More recently, RNA has been implicated in the direct gene regulation of metabolic processes in bacterial cells like Salmonella, subtly adjusting levels of key metabolites, including metal ions.
According to Shi, corresponding author of the new study, such metal ions are critical for Salmonella.
“Not only are they important as enzyme regulators, but also for structural properties," Shi said. "The proper concentration of each metal is very important for bacterial physiology. This is why they need regulation.”
Manganese is of particular concern for researchers, as it plays a central role in the bacterium’s exceptional ability to resist the aggressive (but often ineffective) assault by the host’s defense system.
Tighter regulation
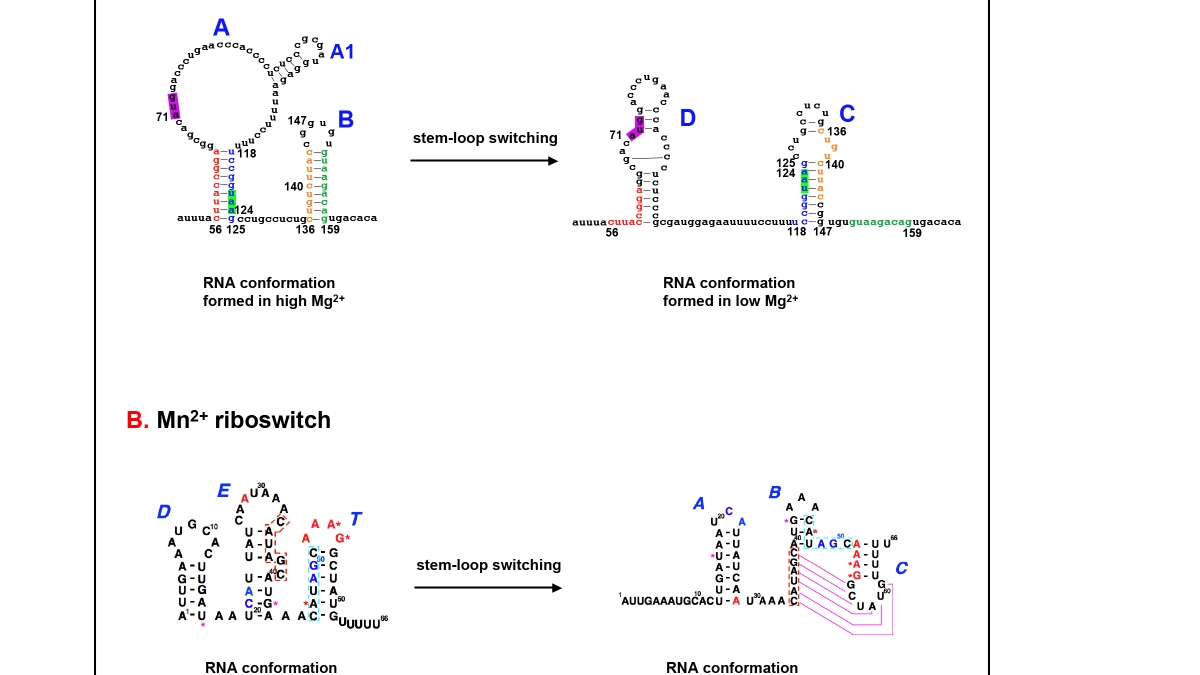
There are two RNA riboswitches described in the study, providing Salmonella with a clever method of rapidly manipulating manganese levels. The first one accurately senses the concentration of manganese ions within the cell, as well as other divalent cations, while the second one is exclusively sensitive to manganese. Both riboswitches impose one of two conformational forms to the RNA strand, thereby raising or diminishing manganese levels as need dictates.
These riboswitches consist of an aptamer, which binds to a specific small molecule – in this case, manganese – and an expression platform that acts to modify gene expression once the small molecule or ligand binds to the aptamer. (The riboswitch sits in the 5’ untranslated region, or 5’UTR of mRNA, but its activity directs downstream transcription or translation events.)
The two conformational alternatives of RNA represent the riboswitch’s "on" and "off" positions. The switched-on position occurs when Salmonella sense that manganese levels are too low. In this case, the riboswitch folds into a configuration permitting RNA transcription to proceed. This process is followed by the translation of a membrane protein known as the transporter, which is responsible for ferrying manganese ions into the bacterial cell.
Once manganese concentrations are sufficient, the RNA conformation switches to the "off" position, forming a transcription terminator, thereby turning off production of the transporter.
The riboswitch can act in several ways to abort the transcription phase of transporter production. As Shi explains, precise ion regulation is critical. “If manganese concentration is too high, it will harm the bacteria because it’s highly toxic,” he says. “It’s really a dance.”
Stealth warfare
The study implies another “dance” – one that takes place between the invasive Salmonella and a particular type of host immune cell known as a macrophage. When the immune system detects the presence of foreign Salmonella bacteria, it uses macrophages to engulf and destroy the invader.
Macrophages have a manganese transporter similar to that found in Salmonella, but use it to pump out the essential metal ion from the bacterium’s new environment. “Host cells try to kill Salmonella through starvation,” co-author Kong explains, referring to this depletion of manganese.
Salmonella, however, fights back, using its own transporter proteins to restore manganese concentrations under the control of an RNA riboswitch. It’s a battle for survival.
The RNA manganese regulator described in the current study is the second riboswitch that can respond directly to the presence of metal ions. In earlier work, they reported the translation-coupled regulatory mechanism of a riboswitch used by Salmonella to oversee intracellular levels of magnesium.
A keener sense
The magnesium riboswitch and the newly discovered manganese riboswitch differ in one crucial respect: while the magnesium riboswitch is also capable of sensing and responding to other ions (including manganese and calcium), the manganese riboswitch is single-minded, responding only to manganese.
The authors suggest that the two riboswitches may act as rough-tuning and fine-tuning controls for Salmonella.
“If you turn off the power to your house, there is no power in any room,” Shi says. “But you can also just turn off the lights in one room and leave the rest of the house alone.” In this fashion, multiple riboswitches may act in concert, providing subtle and sophisticated control over a broad range of metabolites. Such processes are not yet well understood.
Horizon line
Beyond their relevance for manganese regulation in Salmonella, riboswitches have a number of plausible applications. They may be used in laboratory studies as reporter molecules, capable of monitoring intracellular concentrations of metal ions or other molecules. Riboswitch architectures may provide clues for new drugs, allowing researchers to switch off disease pathways.
Perhaps most excitingly, the design of artificial riboswitches or individual components may open an entirely new approach to combating multi-drug resistant pathogens, which have become a major hazard to society.
One line of attack could take advantage of the fact that a natural riboswitch’s aptamer domain tends to be stable and highly conserved over evolutionary time. By engineering an analog ligand – one that the bacterium can’t metabolize – it may be possible to shut down expression of riboswitch-regulated genes.