New imaging technology peers into 3-D world of living single cells
Developed by ASU inventors, the new technology could advance disease diagnostics
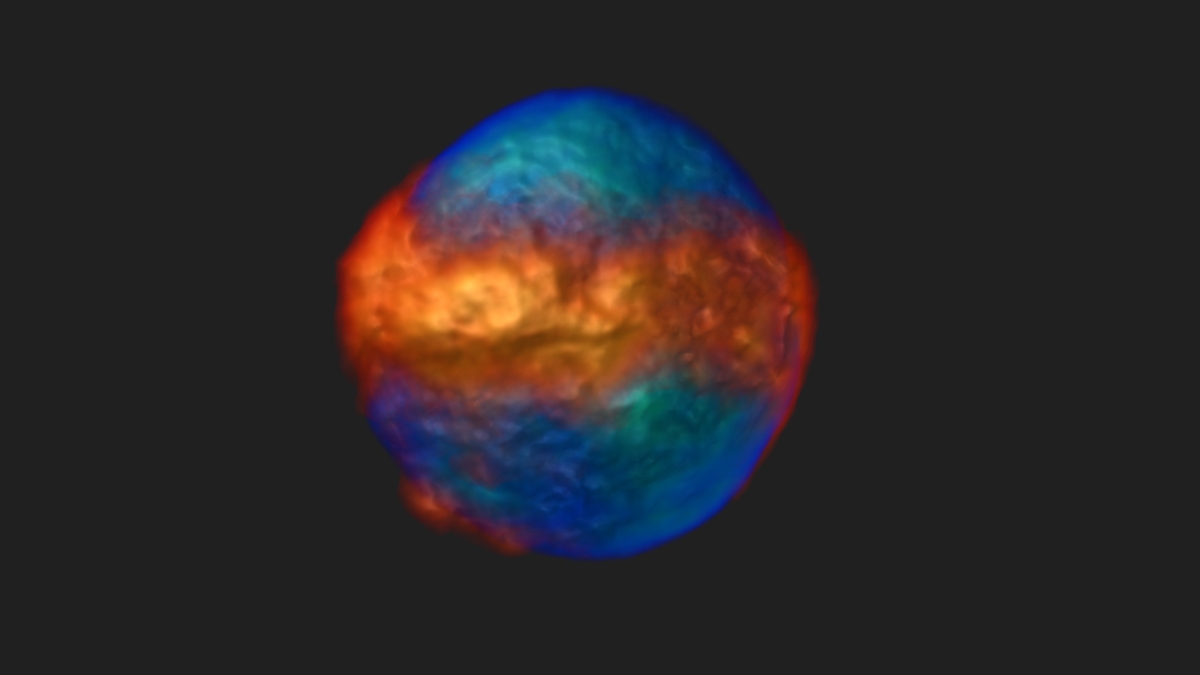
A 3-D image of a live human myelogenous leukemia cell stained for the nucleus (blue-green) and mitochondria (orange-red). The image shows mitochondrial network distribution with respect to the nucleus. The 3-D image was computationally reconstructed from a set of 2-D projections taken on a novel computed tomography-based platform.
Medical imaging technology such as MRIs and CT scans revolutionized the way patients are diagnosed with injuries and disease by enabling doctors to non-invasively look beneath the patient’s skin.
Now, a new technology — called live-cell, CT (or LCCT) — that was recently developed by a team of Arizona State University inventors may soon propel discoveries for the biomedical research community by enabling biologists to explore the inside of living, single cells.
With a premise that the very root of most disease begins within a single cell, the advance of LCCT promises to usher in a new imaging tool important in the early diagnosis of cancer and in fostering a better understanding of cellular processes in cancer and many other diseases.
“Because of LCCT’s capability to perform imaging of live, suspended cells and the relative ease of implementation, we expect the LCCT method to become a powerful new tool for the biomedical research community,” said Deirdre Meldrum, project leader and co-inventor of the new technology, director of the Biodesign Biosignatures Discovery Automation at ASU’s Biodesign Institute, and professor in the Ira A. Fulton Schools of Engineering.
And the ASU innovations may be coming to a clinic sooner than one might think; it was designed from the start with biomedical translation in mind.
“One of our main goals was to design an LCCT system to advance toward clinical and commercial applications in any research or clinical laboratory,” Meldrum said.
World in motion
LCCT imaging is a powerful new tool because it can highlight the key architectural features of single living cells much like MRIs and CTs have done for our bodies.
In this case, think of each cell as an unknown planet where only its surface features were known. Now, with LCCT technology, they are ripe for exploration to reveal their innermost secrets.
At the heart of the technology, the optical live cell CT approach is based on acquiring 2-D images from different perspectives of the cell, like moving a camera around to take pictures from a 360-degree rotational axis.
Each 2-D image has the same resolution (known as isotropic resolution) and can be computed into the 3-D image of the cell with high fidelity and high spatial resolution. The fact that this resolution is the same along all three spatial dimensions is what empowers researchers to interrogate the dynamics of cells in the disease process.
“We wanted to develop a robust technology where we look at the global, 3-D architecture of a cell in its natural state, which is pivotal for the next level of analysis of cellular function and measuring responses to external stimuli or stressors,” Meldrum said.
With that in mind, Meldrum’s team first wanted to demonstrate proof-of-concept of their technology in two important cell biology areas that are revolutionizing medicine: immune system and cancer cells, which may be tipping the scales in the fight against cancer with immunotherapies.
In the first black and white video, LCCT shows much greater detail of cells than the current confocal microscopy studies. The color videos show structural details of two key areas of the cell: the brains (nucleus) and the powerhouse (mitochondria). LCCT can measure these changes to identify a particular cell type, or how drug treatment or diseases may change these cellular features.
On the main stage
To best see the cells in 3-D, they had to find a way to carefully spin them around without damaging them.
The cell rotation issue was solved by placing the cell inside of a 3-D high-frequency electric field. The electric field creates a torque on the cell turning it slowly at speed of 1 to about 3 revolutions per minute. Typically, 300 to 500 projections can be collected from just one full rotation (360°), which are then used to reconstruct the 3-D image.
“We found that the electric field approach produced the most satisfying results in terms of stability, rotation speed, and minimal cell stress,” said Laimonas Kelbauskas, an assistant research professor at the Biodesign Institute who co-invented the technology, conceived and designed the study, and co-developed the technological platform and image reconstruction software.
By putting the cells into their “electrocage” they were able to precisely rotate live cancer and immune cells. Next, after establishing a reproducible and stable cell rotation, they performed a series of imaging experiments with live leukemia and immune sentinel cells known as macrophages, with the goal of characterizing the spatial and temporal resolution limits of LCCT.
They used standard fluorescent dyes that glow when excited by a light source to reconstruct 3-D images and time-lapse movies of single cells. With the dyes, they could see depictions of segmentation of the nuclear and mitochondrial features of the cell.
Mighty mites
Next, they treated the cells with a chemical that is known to prevent segregation of the cell’s mitochondria, and observed the changes they could see in the nucleus.
Every 30 seconds, they were able to resolve structural details of the cell that are about 300 times smaller than the diameter of a human hair.
They found striking changes to the shape of the mitochondria that had never been seen before.
“We believe that this is the first report of immune system cells imaged in their natural state — suspended in aqueous medium, as opposed to attached to a glass slide — with fully isotropic 3D spatial resolution,” Meldrum said. “Our experimental findings indicate that a majority of mitochondria in suspended cells are highly interconnected, forming a complex and dynamic filamentous network.”
The pill-shaped mitochondria had previously been thought to be independent operators, and showing the evidence of an interconnected network may cause cell biologists to rethink the ways mitochondria work within the cell.
“We, therefore, conclude that the observed mitochondrial morphology can be attributed to the cell type specificity and/or the fact that the cells are imaged suspended in 3-D space rather than adhered on a planar substrate. The conventional 2-D imaging of cells on glass slides may become obsolete as 3-D imaging of cells in suspension supersedes the 200-year old approach of 2-D conventional microscopy,” Meldrum said.
In addition, because they measure and see precise changes within the cell, it may open up LCCT technology to identify the exact cell types found within cancer cells that lead to metastasis, or the immune response to infectious agents. This may lead to better and more targeted disease therapies.
“The quantification of mitochondrial and nuclear remodeling over time enables direct multiparameter comparisons between individual cells or cell types, offering a unique way for rare cell identification with high accuracy,” Kelbauskas said.
Next steps
For the next series of experiments, the team will expand on their studies to look at more cell types. They can expose cells to environmental stressors, measure responses to drug treatments and use different imaging methods to further improve the spatial and temporal resolution of the technology.
The Meldrum team will also continue to collaborate with industry toward clinical impact.
“In summary, we feel the LCCT is a breakthrough, a powerful and versatile tool for investigating individual cellular dynamics and enabling quantitative studies of cellular architecture dynamics, and could be used for combined nuclear and mitochondrial organization studies in response to different treatment regimens,” Meldrum said.
This work was supported by a grant from the W. M. Keck Foundation (024333-001).
More Science and technology

The science behind chronic stress
Stress comes in many shapes and sizes. There’s the everyday stress of preparing for a final exam or being stuck in traffic. And…

ASU planetary scientist to be inducted into the National Academy of Sciences
The National Academy of Sciences is inducting School of Earth and Space Exploration Director Meenakshi Wadhwa into the 2023 class…

Unlocking the potential of AI for homeland security
“Can we do what we're doing now cheaper, more efficiently, more effectively?” Adam Cox, director in the Office of Strategy and…


